Appearance
Pill Connect User Manual
Foreword
Read these instructions completely before operating this device. Proper training is required prior to use and should cover all features, operating conditions, and troubleshooting procedures.
CAUTION
- This device is NOT intended for monitoring, treating, or diagnosing any disease or medical condition.
- The Pill Connect Dispenser's sole function is to dispense pills upon user instruction and record the timestamp of each dispensing event.
- All clinical instructions—including medication regimen, dosage, and timing—remain the exclusive responsibility of you and your healthcare team.
- The Pill Connect Dispenser does not provide guidance on medication quantity or administration timing. Always follow your prescribed medication regimen as directed by your clinical or care team.
Quick start guide
Watch this instructional video for a quick overview of the Pill Connect Dispenser.
Scan this QR code to watch the video
https://vimeo.com/1072553754/3e51547b59
How to use the Pill Connect Dispenser

Attach dispenser
Firmly press down and twist clock-wise to secure the dispenser to your medication bottle. If this is your first use, the light may briefly flash white after a few seconds to indicate the system has powered on.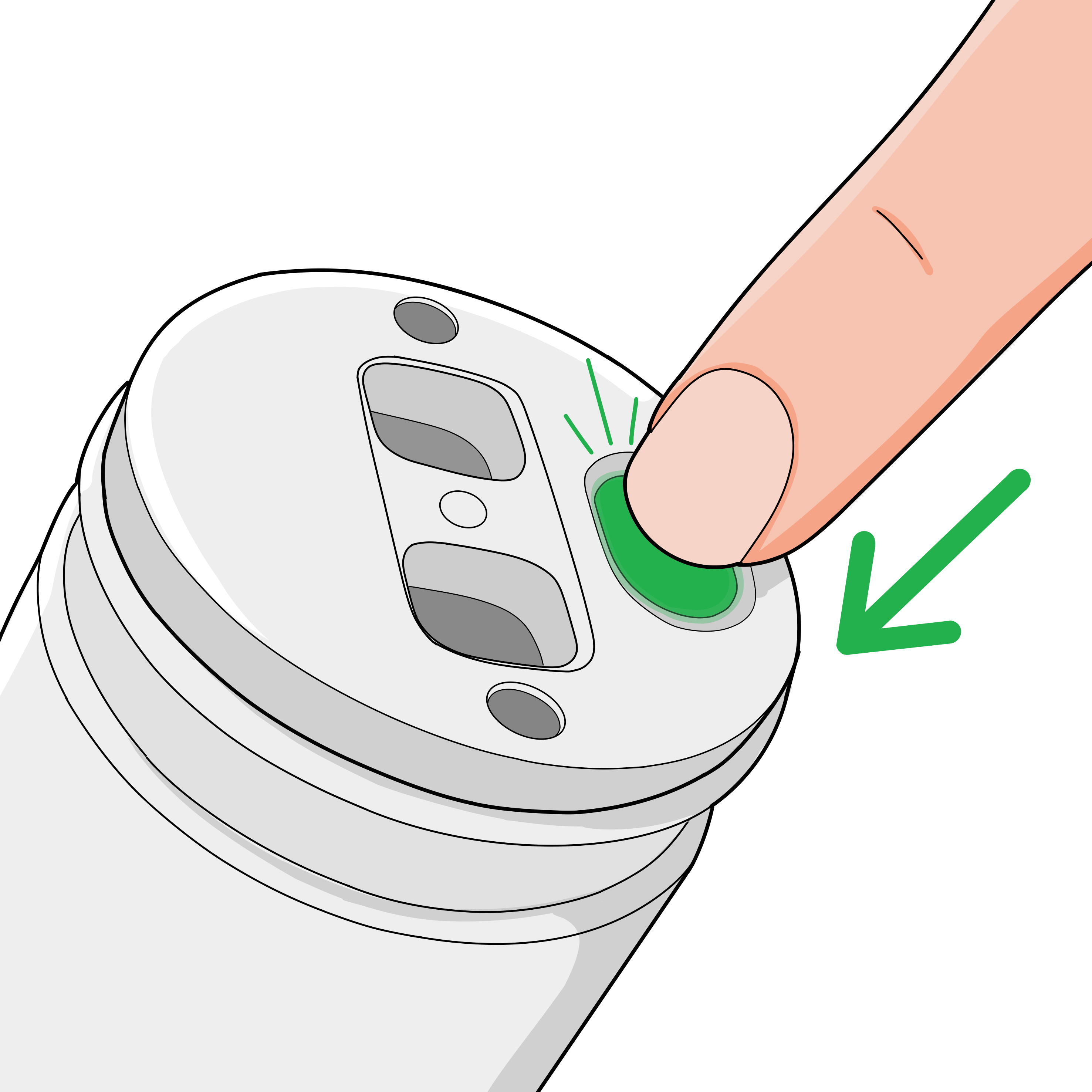
Press button
Remove the protective cap and press the green button on top of the device to initiate dispensing.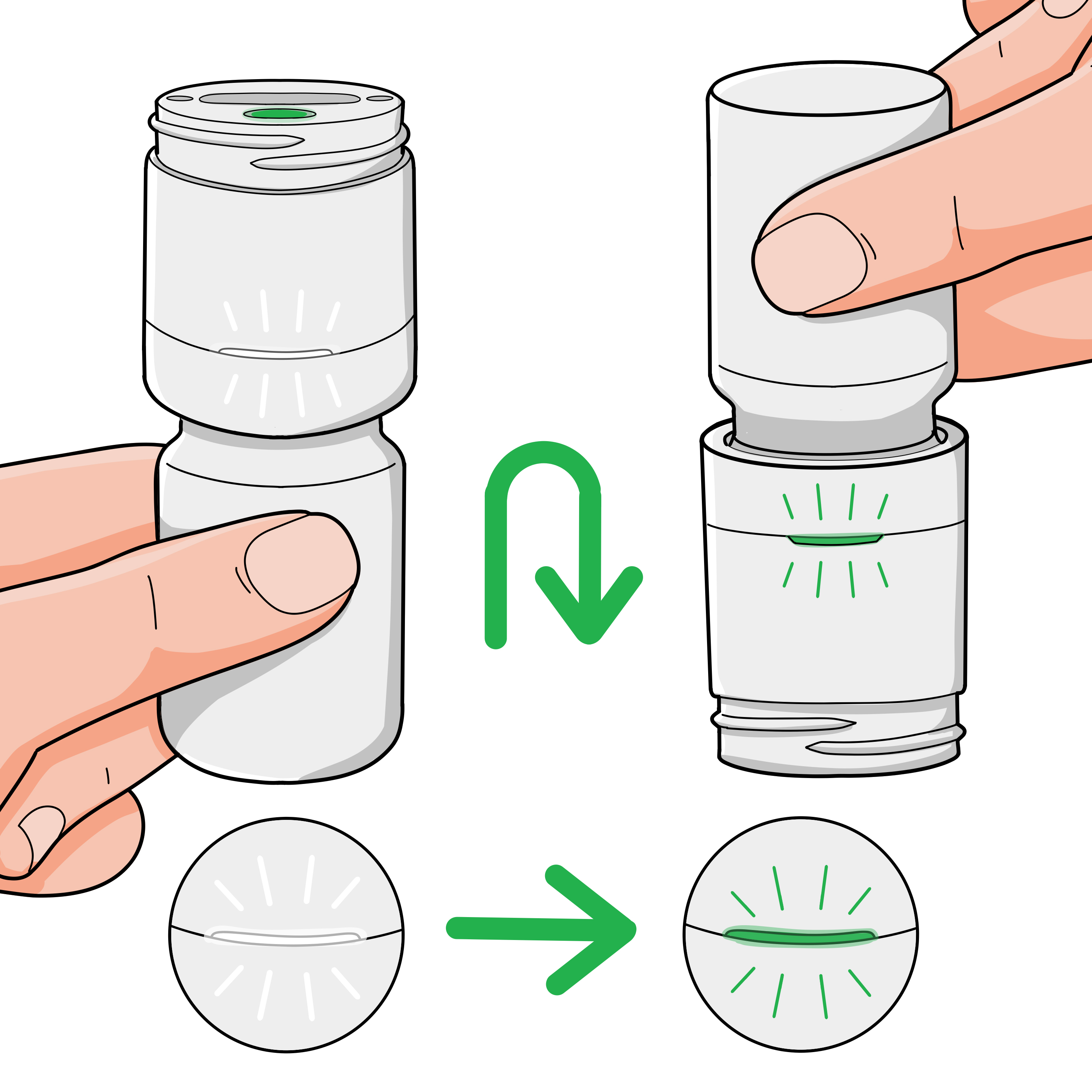
Turn upside down
When the status light begins flashing white, turn the bottle completely upside down. This position allows the medication to dispense from the device.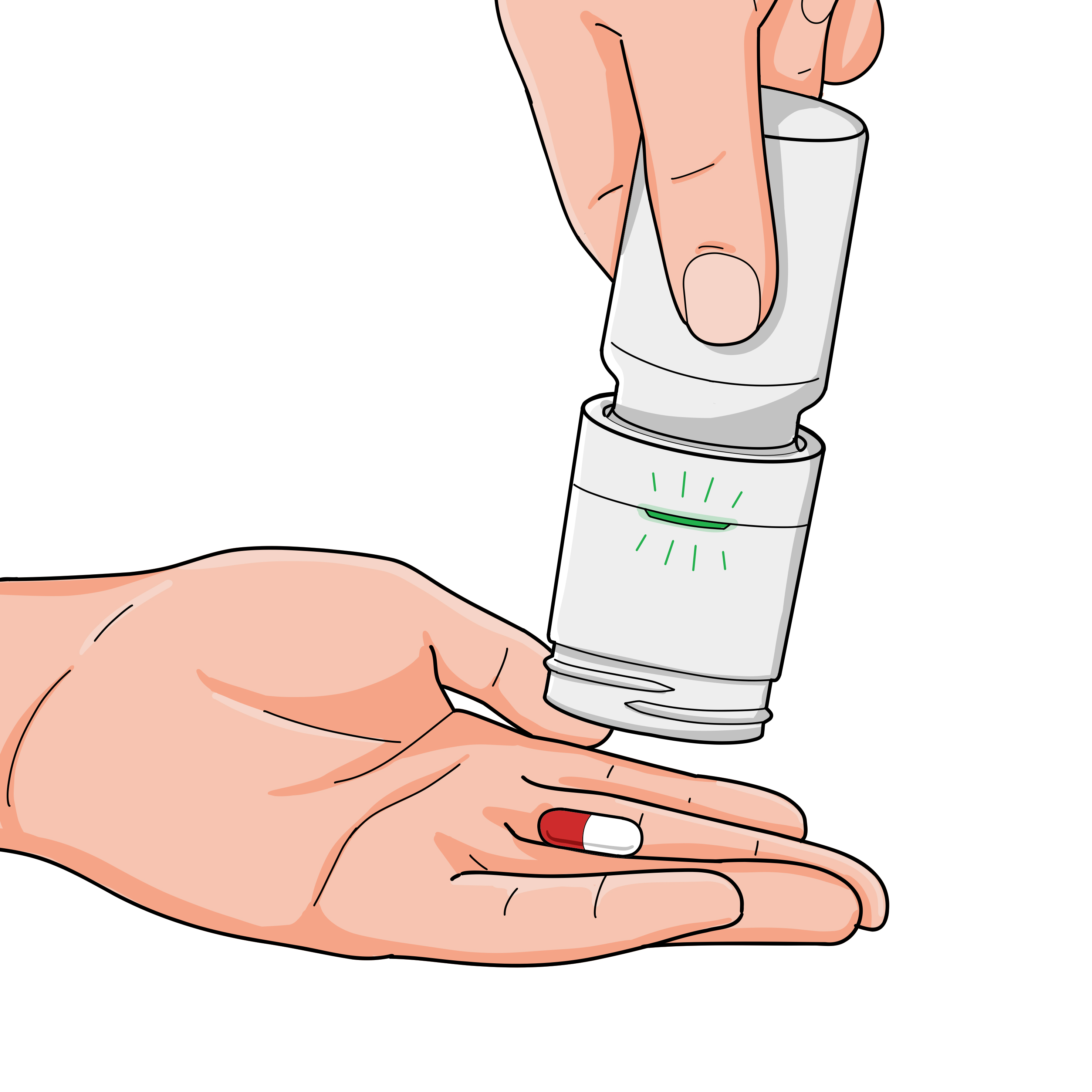
Collect pill
While holding the device upside down, the status light will change to flashing green. Position your hand beneath the device to catch the medication when it dispenses.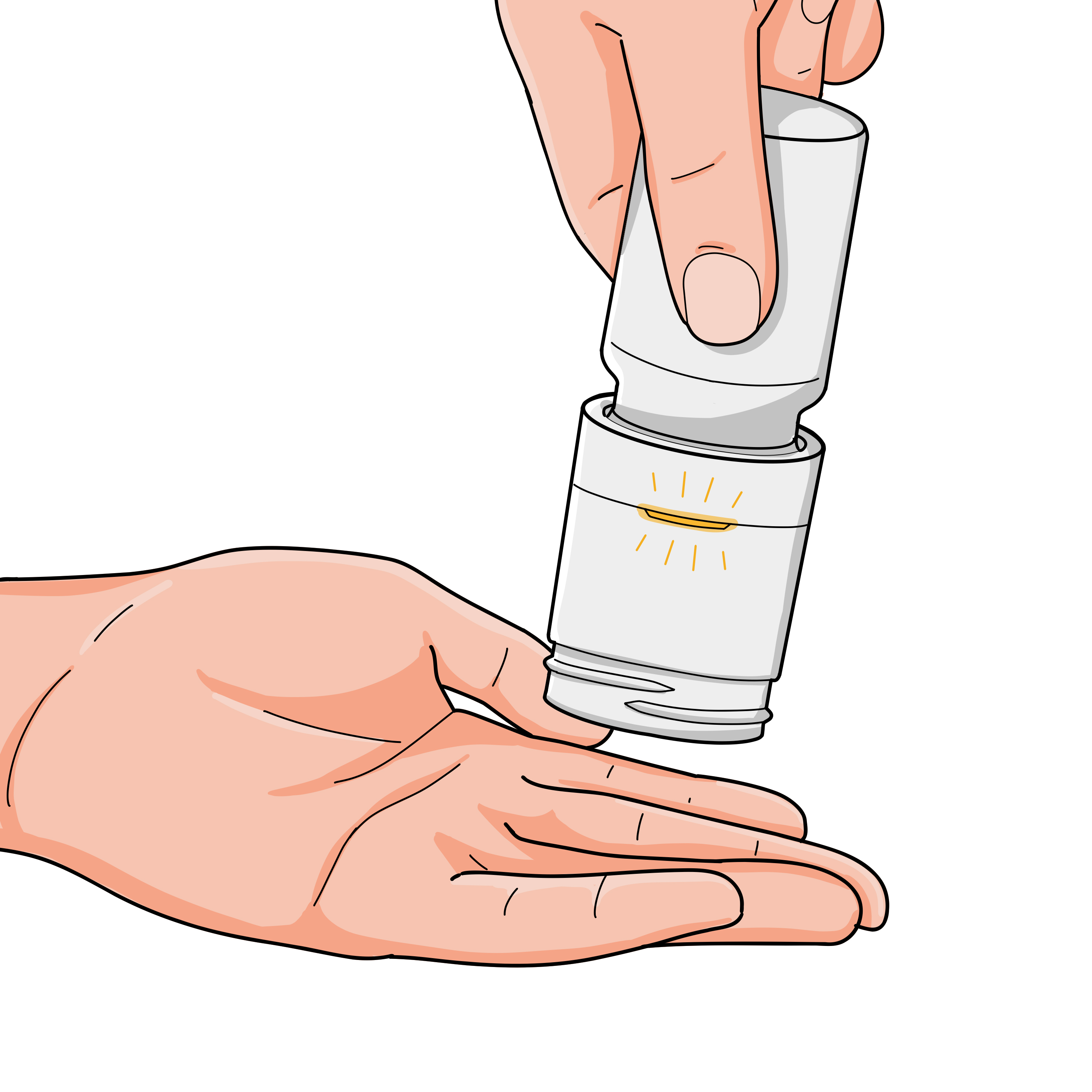
No pill dispensed
If no pill is dispensed following the steps above you will see an orange light. Confirm there are pills remaining in your bottle and try again.Detailed operating instructions
Device components
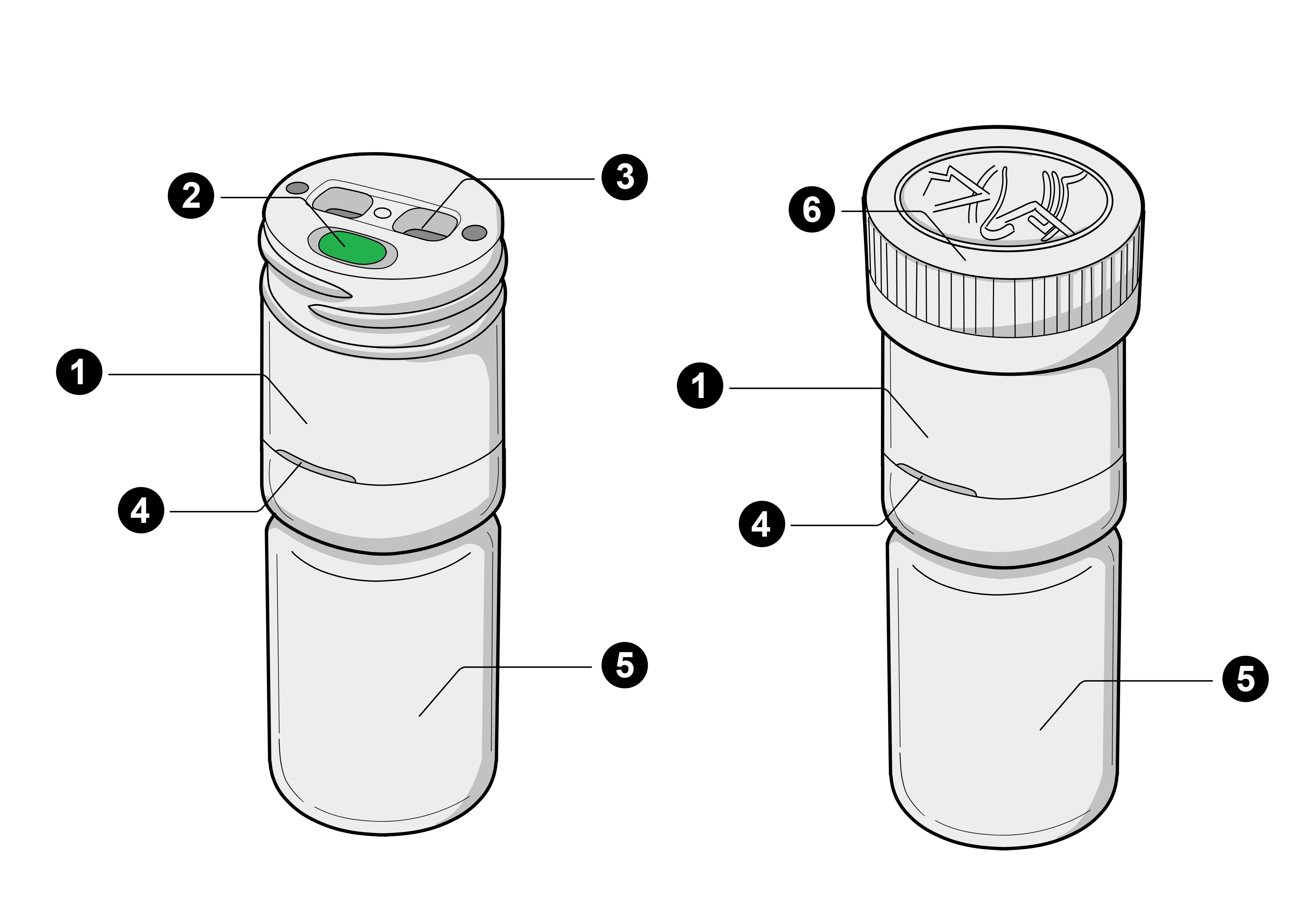
Components of the Pill Connect Dispenser
| Ref | Component | Description |
|---|---|---|
| 1 | Dispenser | The Pill Connect Dispenser |
| 2 | Button | Pressing this button begins the medication dispensing process |
| 3 | Exit Chutes | Openings where the medication is dispensed from the device |
| 4 | Status Light | Displays device status through colour-coded light signals |
| 5 | Bottle | Standard bottle that attaches to the dispenser containing the medication |
| 6 | Protective Cap | Child-resistant cap that covers the dispenser when note in use |
Attaching and removing the Dispenser from a Bottle
The Dispenser attaches to and removes from a medication bottle using a standard child-resistant push-and-twist mechanism.

To attach:
Push down on the dispenser and turn clock-wise until secure. Avoid overtightening.
Verify proper attachment by attempting to turn counter-clockwise without pushing down. A clicking sound indicates the child-resistant mechanism is engaged.
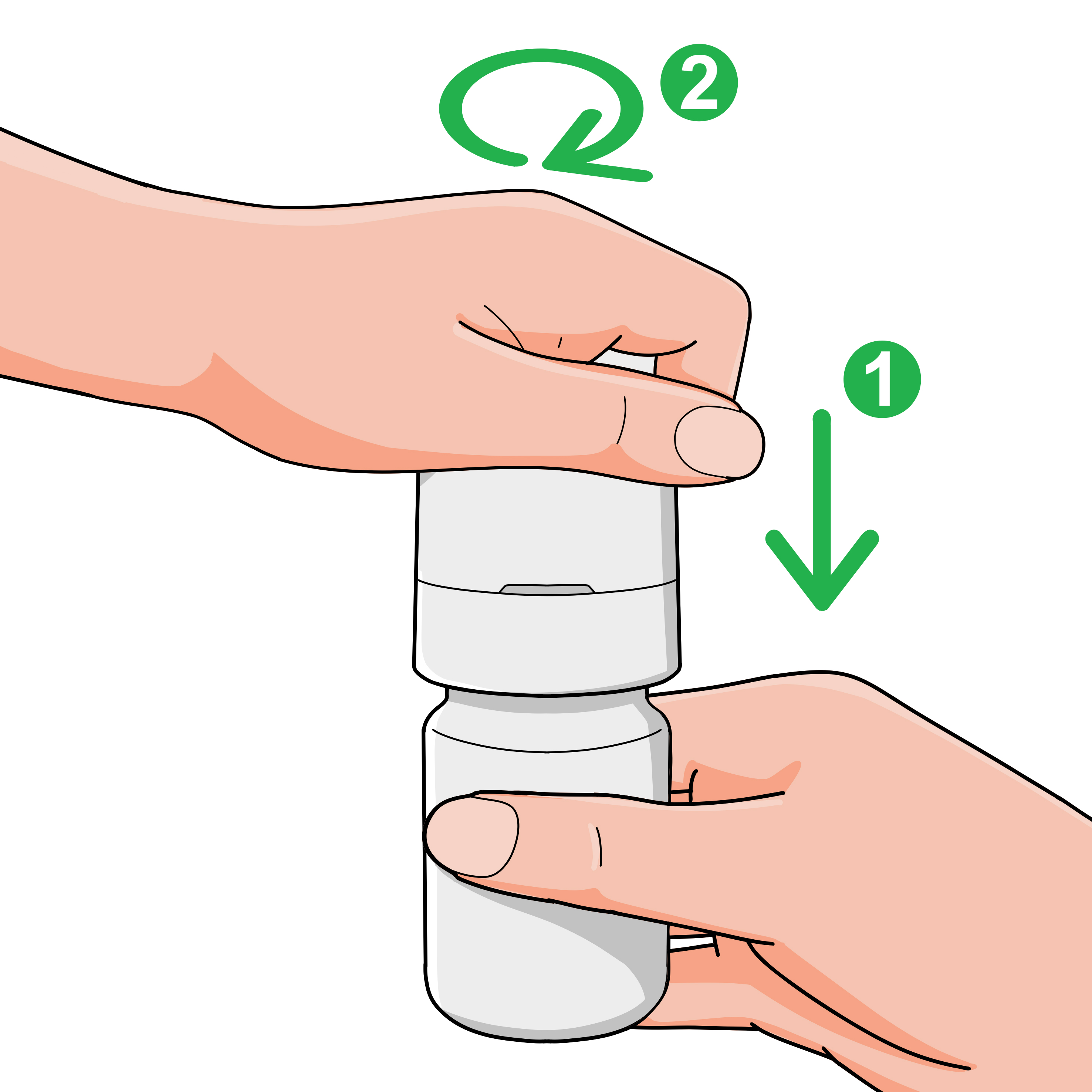
To remove:
- Hold the bottle securely, push down on the dispenser, and turn counter-clockwise until released.
Removing the top cap from the Dispenser
The top cap uses a child-resistant push-and-twist mechanism similar to the dispenser.
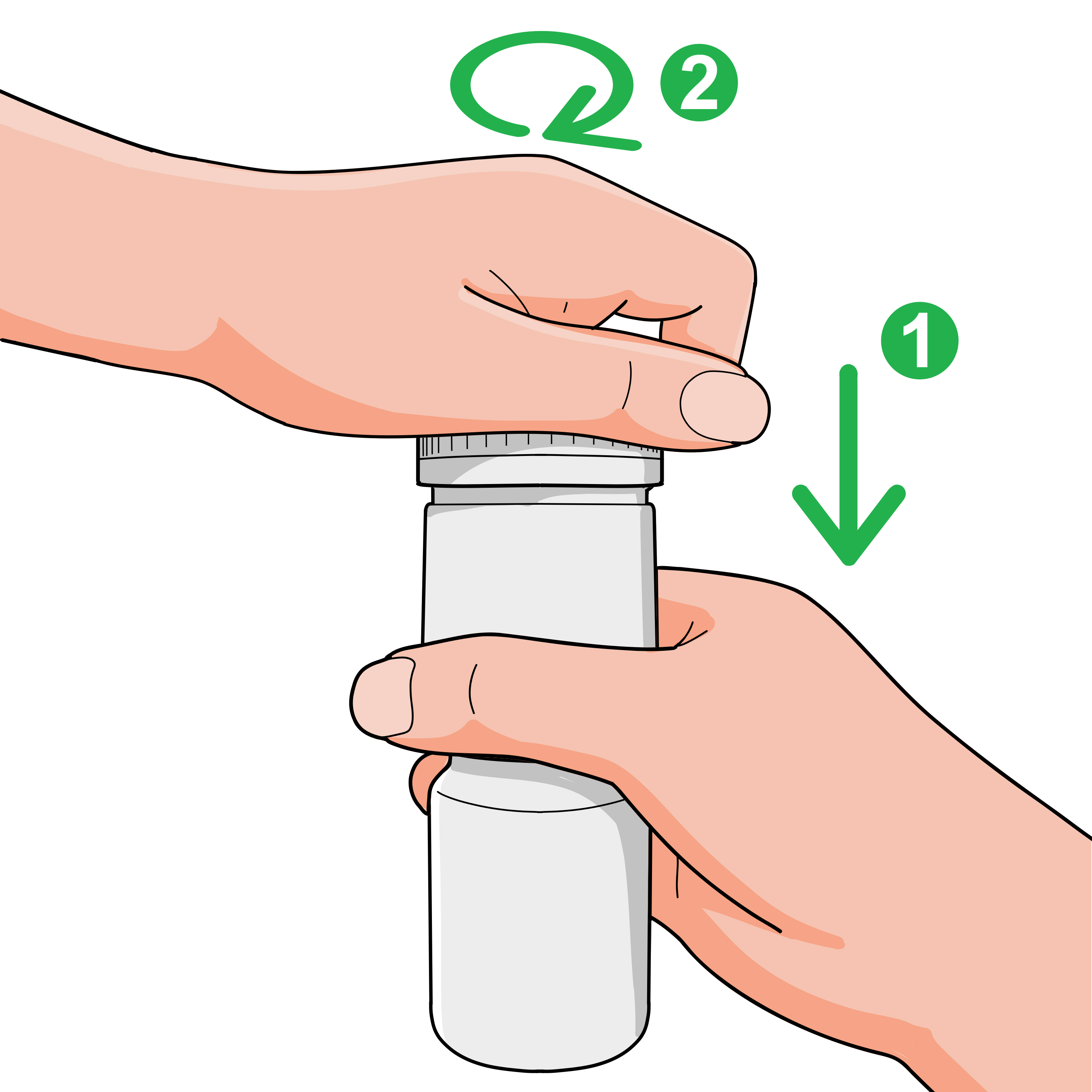
To remove:
- Hold the dispenser securely, push down on the cap, and turn counter-clockwise until released.
Refills
CAUTION
Your dispenser is designed specifically for use with the medication provided by your clinical trial team. Do not use this dispenser with any other medication.
For complete refill instructions, please contact your trial coordinator. Instructions for removing the dispenser from the bottle can be found above.
Cleaning and decontamination instructions
CAUTION
Never immerse the dispenser in water or other liquids. The device contains sensitive electronics that may be damaged by moisture.
To maintain optimal performance and hygiene of your Pill Connect Dispenser:
- Remove the dispenser from the medication bottle following the instructions above.
- Wipe all exterior surfaces with a clean, slightly dampened lint-free cloth.
- For sanitizing the device, use a cloth lightly moistened with 70% isopropyl alcohol.
- Allow the dispenser to dry completely before reattaching to a medication bottle.
- Do not use abrasive cleaners, solvents, or chemical sprays as these may damage the device.
Storage recommendations
Proper storage of your Pill Connect Dispenser ensures optimal performance and extends device life.
Recommended storage conditions
- Temperature: Store between 0°C and 45°C (32°F – 113°F)
- Humidity: Maintain in environments with relative humidity between 20% and 80% (non-condensing)
- Environment: Keep in a clean, dust-free location away from direct sunlight and heat sources
- Handling: When not in use, ensure the protective cap is securely attached to prevent contamination
End of life disposal
When the device battery depletes or you no longer need the device, return the Pill Connect Dispenser to your clinical trial team for proper disposal.
CAUTION
Do not dispose of this device in regular household waste. The dispenser contains electronic components and a battery that require specialized disposal procedures in accordance with local regulations for electronic waste.
Battery information
WARNING
This product contains a Lithium Battery. Risk of fire, explosion, or chemical burn if mishandled. The battery is integrated and non-user replaceable. Do not attempt to open, disassemble, or modify the device under any circumstances.
The Pill Connect Dispenser contains a non-replaceable lithium battery with the following specifications:
- Battery Type: Integrated lithium battery
- Expected Battery Life: Approximately 12 months under normal usage conditions
- Battery Disposal: Return the entire device to your clinical trial coordinator for proper disposal when battery is depleted
Important information and environmental operating conditions
- Air travel: Verify battery transportation restrictions before traveling with this device on aircraft.
- Operating temperature range: 4°C to 40°C (40°F – 104°F).
- Device integrity: Discontinue use immediately if the device shows any signs of damage and contact your trial team.
- Moisture protection: Keep the device dry. Avoid exposure to water and humid environments.
- Safety: Store the device out of reach of children at all times.
Troubleshooting
The table below addresses common issues encountered during pill dispensing operations. If you are unable to resolve your issue using these troubleshooting steps, please contact your site trial team immediately.
| # | Problem | Solution |
|---|---|---|
| 1 | I press the button and see the white light flash, but nothing happens. | The dispenser must be held upside down for pills to come out, try flipping the device and holding your hand out below to catch the dispensed pill. |
| 2 | I press the button, turn it upside down and see the green light, but no pill comes out and I see the light flash orange. | Sometimes, pills need encouragement to dispense. When you press the button and turn the bottle upside down, give the bottle a small shake when the light is green. |
| 3 | I press the button and the light does not flash, no pills are dispensed. | Contact your site team. |
| 4 | My pill bottle is empty, how do I replace it? | Follow the instructions above for detaching the dispenser from the bottle. Contact your trial team for guidance on refills. |
| 5 | I can't remove the lid to access the pills. | The bottle has a child resist mechanism to prevent children accessing medicine. You can remove the lid with a push and twist motion as shown in above. |
| 6 | My dispenser has visible damage or does not look safe to use, what should I do? | Remove the dispenser. You can continue to access the pills directly from the bottle. Contact your site team. |
| 7 | I wish to access my pills without the dispenser. | You can remove the dispenser from the bottle following the instructions above. If you are finding difficulties with the dispenser, contact your trial team and share your concerns. |
| 8 | I wish to return a pill to the bottle. | To return a pill, remove the dispenser from the bottle following the instructions above. Return the pill(s) to the bottle then reattach the dispenser as shown in the instructions above. Notify your trial team if you have returned pills to the bottle after dispensing. |
Regulatory information
This device is authorized for use in the United States and Canada only. See country-specific labeling information below.
United States of America
Contains FCC ID: T7V1780
The device meets the requirements for modular transmitter approval as detailed in FCC public Notice DA00-1407. The transmitter operation is subject to the following two conditions:
- This device may not cause harmful interference, and
- This device must accept any interference received, including interference that may cause undesired operation
CAUTION
The FCC requires the user to be notified that any changes or modifications made to this device that are not expressly approved by Panasonic Industrial Devices Europe GmbH may void the user's authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception (which can be determined by turning the equipment off and on) you can try to correct the interference by one or more of the following measures:
- Reorient or relocate the receiving antenna,
- Increase the separation between the equipment and receiver,
- Connect the equipment into an outlet on a circuit different from that to which the receiver is connected,
- Consult the dealer or an experienced radio/TV technician for help.
CAUTION
To comply with FCC RF Exposure requirements, the OEM must ensure that only antennas from the Approved Antenna List are installed.
| Part Number | Manufacturer | Frequency Band | Type | Max. Gain (dBi) |
|---|---|---|---|---|
| ANT016008LCS2442MA1 | TDK | 2.4Ghz | Chip Antenna | -1.0 |
Canda
Contains IC: 216Q-1780
INFO
The device PAN1780, including the integrated antenna mentioned in the Approved Antenna List above, complies with Canada RSS-GEN Rules. The device meets the requirements for modular transmitter approval as detailed in RSS-Gen.
Operation is subject to the following two conditions:
- This device may not cause harmful interference, and
- This device must accept any interference received, including interference that may cause undesired operation
L’ identifiant IC est: IC: 216Q-1780
INFO
Le présent appareil PAN1780, les antennes y compris Approved Antenna List est conforme aux CNR-Gen d'Industrie Canada applicables aux appareils radio exempts de licence
L'exploitation est autorisée aux deux conditions suivantes:
- L'appareil ne doit pas produire de brouillage, et
- L'utilisateur de l'appareil doit accepter tout brouillage radioélectrique subi, même si le brouillage est susceptible d'en compromettre le fonctionnement
Technical specifications
General specifications
| Specification | Details |
|---|---|
| Model Name | Pill Connect Dispenser |
| Device Classification | Electronic pill dispenser with tracking capabilities. |
| Product Type | Medication management device. |
| Manufacturer | Pill Connect Limited |
| Country of Origin | United Kingdom |
| Serial Number | Refer to device label. |
| IP Rating | N/A |
| Use Environment | Designed primarily for use indoors. |
Environmental operating conditions
| Parameter | Operating range | Storage range |
|---|---|---|
| Temperature | 4°C to 40°C (40°F to 104°F) | 0°C to 45°C (32°F to 113°F) |
| Relative Humidity | 20% to 80% (non-condensing) | 20% to 80% (non-condensing) |
| Altitude | Up to 2000m above sea level. | Up to 2000m above sea level. |
| Atmospheric Pressure | 70 kPa to 106 kPa | 70 kPa to 106 kPa |
Electrical specifications
| Parameter | Specification |
|---|---|
| Power Source | Internal non-replaceable lithium battery. |
| Battery Type | Lithium Polymer |
| Battery Voltage | 3.7V nominal |
| Battery Capacity | Suitable for approximately 12 months of normal use. |
| Maximum Power Consumption | < 100mW during dispense. |
| Standby Power Consumption | < 75μW |
Wireless specifications
| Parameter | Specification |
|---|---|
| Frequency Band | 2.4 GHz ISM Band (2402-2480 MHz) |
| Maximum RF Output Power | < 8dBm |
| Antenna | Integrated chip antenna (TDK ANT016008LCS2442MA1) |
| Antenna Gain | -1.0 dBi |
| FCC ID | Contains FCC ID: T7V1780 |
| IC ID | Contains IC: 216Q-1780 / Contient IC: 216Q-1780 |
Mechanical specifications
| Parameter | Specification |
|---|---|
| Dimensions (Dispenser Only) | Approximately 47x47x74mm |
| Weight (Dispenser Only) | <100g |
Manufacturer information
Support Contact
For all support inquiries, please contact your trial site team first. They will help coordinate with Pill Connect if necessary.
Pill Connect Limited, 125 Deansgate, Manchester, M3 2BY, UK.
